In this article
Meet the experts
Dr. Kyle Hoedebecke, MD, MBA, MPA, MS, FAAFP, CPE, is a Clinical Advisor at Alpas Wellness.
Sita Severson is a CIAYT‑certified yoga therapist and educator who trains teachers in yoga therapy, pranayama, and Ayurveda. She directs advanced training and leads evidence‑informed programs that honor Yogic roots while meeting modern needs.
Gerome Burke, M.D., Ph.D., is a physician, medical toxicologist, and expert contributor at Drugwatch.com.
Alison Swiggard, MS, RDN, LD, is a registered dietitian at CV Wellbeing.
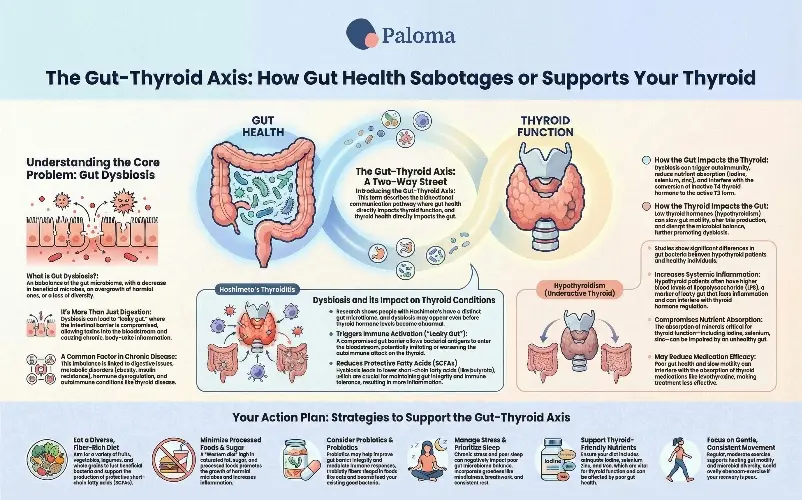
Your gut microbiome, comprising trillions of bacteria, fungi, viruses, and other microbes, is a dynamic environment that plays a pivotal role in maintaining overall health. In a state of balance, this complex community, known as the gut microbiota, assists in nutrient absorption, synthesizes essential vitamins, trains and regulates your immune system, and helps protect against pathogens. However, when this balance is disrupted – characterized by a decrease in beneficial microbes, an overgrowth of harmful ones, or a significant loss of microbial diversity – the condition is termed gut dysbiosis.
Gut dysbiosis is not merely a digestive inconvenience; it can have far-reaching effects. It can compromise the integrity of your intestinal barrier, leading to a phenomenon often referred to as “leaky gut” or increased intestinal permeability. This compromised barrier allows undigested food particles, toxins, and microbial byproducts to enter the bloodstream, triggering a cascade of chronic inflammation and immune system dysregulation. This pervasive inflammation can affect virtually any organ system, including the thyroid gland, which is a critical regulator of metabolism and energy. The gut-thyroid axis, a bidirectional communication pathway, means that imbalances in the gut can directly impact thyroid function. Conversely, thyroid dysfunction can further alter the gut environment, creating a challenging cycle.
In short: a healthy gut microbiome is foundational — and gut dysbiosis can contribute to a host of health problems, from digestive issues to metabolic and immune disorders. Let’s explore gut dysbiosis and how it affects your thyroid, hormones, and weight-loss efforts.
Gut dysbiosis is linked with many different conditions. Some of the most common associations include:
- Digestive issues — bloating, gas, constipation or diarrhea, increased intestinal permeability.
- In autoimmune or inflammatory diseases, dysbiosis has been observed beyond the gut, e.g., in thyroid autoimmunity.
- Metabolic disorders — obesity, insulin resistance, metabolic syndrome, type 2 diabetes.
- Hormone-related dysregulation — because gut microbes influence nutrient absorption, immune signaling, and even hormone metabolism, dysbiosis can disrupt endocrine health.
- Reduced microbial diversity & resilience — a less diverse gut microbiome can be less stable and more prone to overgrowth of harmful species.
In many cases, dysbiosis may not cause disease on its own. Still, it can contribute significantly to disease onset, worsening, or poor recovery, especially when combined with genetic, dietary, or environmental stressors.
Gut dysbiosis often results from a mix of lifestyle, physiological, and environmental factors. Here are the most common:
- Diet: A diet low in fiber and high in saturated fats, sugar, and processed foods — often called a “Western diet” — can reduce the amount of beneficial microbes and increase harmful ones.
- Low intake of prebiotics/fiber: Without enough fiber (the fuel for many beneficial bacteria), communities lose diversity and beneficial metabolites (like short-chain fatty acids).
- Hormonal changes: Hormone fluctuations — e.g., during perimenopause, menopause, or thyroid dysregulation — can alter the gut environment and microbial balance.
- Chronic inflammation or immune dysregulation: Ongoing inflammation (due to stress, infections, autoimmune conditions, poor gut barrier) can disrupt microbiome balance.
- Thyroid dysfunction: Because thyroid hormones influence gut motility, bile production, and the gut–microbiota interplay, hypothyroidism or other thyroid conditions can, in turn, promote dysbiosis.
- Aging, menopause, sex hormone decline: In women, declining estrogen/progesterone seen in perimenopause and menopause appear to be linked to changes in the gut microbiome.
Research has shown that gut dysbiosis and Hashimoto’s thyroiditis – the autoimmune disease that is the most common cause of hypothyroidism in most industrialized nations -- are connected.
- A 2022 study comparing people with Hashimoto’s versus healthy controls found differences in gut microbiota composition. Certain bacteria were more prevalent in Hashimoto’s patients (e.g., Akkermansia, Bifidobacterium, some Lachnospiraceae), while others (e.g., Lachnoclostridium, Bilophila, Klebsiella) were more common in healthy controls.
- A 2024 analysis looked at people with early Hashimoto’s (positive thyroid antibodies but normal thyroid hormones) and found subtle but measurable shifts in gut microbiome composition — suggesting dysbiosis may begin even before overt hypothyroidism.
- The broader literature supports the concept of a “gut-thyroid axis,” in which microbiota influence immune regulation, gut permeability, and nutrient absorption (iodine, selenium, zinc)—nutrients crucial for thyroid health.
Gut dysbiosis appears to be able to trigger or worsen Hashimoto’s. There are four prevalent theories regarding the mechanisms.
- Leaky gut → immune activation: Dysbiosis may impair the gut barrier, allowing bacterial antigens to cross into the bloodstream, which triggers immune responses. In genetically predisposed individuals, this may help initiate or worsen an autoimmune thyroid attack.
- Reduced single-chain fatty acid (SCFA) production: SCFAs (such as butyrate) produced by healthy gut bacteria support intestinal integrity and immune tolerance. Dysbiosis leads to fewer SCFA producers, which leads to an impaired gut barrier and a pro-inflammatory immune state.
- Altered hormone & nutrient metabolism: Gut microbes influence the absorption and metabolism of iodine, selenium, and other micronutrients — which are vital for thyroid hormone synthesis and regulation.
- Cytokines, bile acids, immune signaling: Dysbiotic microbiota can shift cytokine and secondary bile acid production, affecting immune regulation and possibly promoting autoimmunity.
Gut dysbiosis and hypothyroidism – an underactive thyroid – have been shown in research to be connected.
- A 2020 study compared 52 patients with primary hypothyroidism to 40 healthy controls. Using genetic sequencing, researchers found significant differences in gut microbiota diversity and composition.
- Specifically, certain bacteria (e.g., Veillonella, Paraprevotella, Neisseria, Rheinheimera) could reliably distinguish hypothyroid patients from healthy people.
- Hypothyroid patients had a reduced capacity to produce SCFAs – essential for gut health – and increased levels of lipopolysaccharide (LPS) in the blood, a marker of increased gut permeability and systemic inflammation.
- The same study transplanted fecal microbiota from hypothyroid patients into mice — the mice developed lowered thyroid hormone levels! That suggests that an altered microbiome may directly influence thyroid hormone metabolism.
- A 2025 review introduced the concept of the “gut–thyroid axis,” describing how gut dysbiosis can influence thyroid hormone production, metabolism, immune activation, and even hormone tissue sensitivity.
How does dysbiosis contribute to hypothyroidism? There are several pathways.
- Loss of SCFA-producing bacteria can compromise gut integrity and increase systemic inflammation — potentially affecting thyroid hormone synthesis or conversion.
- Increased gut permeability allows LPS and other bacterial byproducts into the circulation, which can activate inflammatory pathways that may interfere with thyroid hormone regulation.
- The gut microbiome influences circulation, deiodinase activity, and hormone conversion, which can disrupt T4-to-T3 conversion or hormone clearance.)
- With gut dysbiosis, absorption of minerals like iodine, selenium, and zinc — all critical for thyroid function — can be compromised.
Scientific research has shown a clear link between hormonal changes during perimenopause and menopause and changes in the gut microbiome.
- A 2022 study comparing women with menopausal syndrome to healthy controls found marked gut microbiota dysbiosis in the MPS group — certain beneficial bacteria (e.g., Bifidobacterium animalis, Acinetobacter guillouiae, Aggregatibacter segnis) were reduced in women with more significant menopausal symptoms.
- An extensive population study of postmenopausal women found that their gut microbiome became more similar to the typical male microbiome after menopause. This shift involved changes in microbial functions related to hormone metabolism (the “estrobolome”), and was associated with adverse cardiometabolic markers.
- A 2021 review on menopause, obesity, and the gut microbiome concluded that declining estrogen/progesterone levels and menopausal changes contribute to reduced microbiome diversity, increased “dysbiosis ratio,” altered bile acid and estrogen metabolism, and increased systemic inflammation.

Why do hormonal changes likely drive dysbiosis? Sex hormones influence your gut motility, mucus production, immune tone, and the gut environment — all of which affect which microbes thrive. As your estrogen levels drop during perimenopause and menopause, those shifts can destabilize your gut ecosystem.
The “estrobolome” — the collection of gut microbial genes that metabolize estrogens — becomes particularly relevant. Changes in which bacteria are present can influence how estrogen is recycled, broken down, or excreted — potentially affecting your systemic hormone balance.
Reduced beneficial microbes (SCFA producers, anti-inflammatory bacteria) plus increased pro-inflammatory microbes may contribute to common menopausal symptoms such as weight gain, metabolic changes, mood problems, sleep disturbances, and hot flashes.
Gut dysbiosis also has an impact on the success of weight loss efforts and can affect the way energy is processed from food. Some microbiome compositions extract more energy (calories) from the same food, promoting fat storage.
- Loss of SCFA-producing bacteria, increased gut permeability, and inflammation can all contribute to insulin resistance, dysregulated metabolism, and fat accumulation. (PubMed)
- Dysbiosis affects the hormones that regulate appetite and metabolism: SCFAs and microbial metabolites help regulate gut hormones (e.g., GLP-1, PYY) and influence energy balance. When dysbiosis is present, this hormonal regulation may be negatively affected. (MDPI)
Sita Severson is a therapist and educator for programs that honor Yogic roots. According to Sita: “Gut dysbiosis makes weight loss harder because the gut is the command center for metabolism. When the microbiome becomes unbalanced, inflammation rises, thyroid hormone conversion becomes less efficient, and insulin signaling becomes sluggish. The body holds weight as a form of self-protection when the digestive system is overwhelmed. In Ayurveda, this is viewed as a loss of digestive fire. Food is not broken down fully, so it ferments and forms heaviness in the body instead of energy. Weight loss resistance in dysbiosis is not a willpower issue. It is the body asking for digestive repair. “
How to address dysbiosis with Hashimoto’s
Probiotic supplements: According to a recent systematic review, supplementing with probiotics may help in thyroid disorders by improving gut barrier integrity, reducing systemic inflammation, modulating immune responses, and improving absorption of micronutrients necessary for thyroid hormone production.
Gut-focused approach: Even in early Hashimoto’s (before the onset of overt hypothyroidism), gut dysbiosis is detectable. This suggests that gut-targeted strategies may help slow progression or mitigate autoimmune activity.
In people with Hashimoto’s and GI symptoms (e.g., bloating, constipation/diarrhea), a gut-focused approach (diet, prebiotics/probiotics, gut-healing strategies) may be particularly beneficial.
How to address dysbiosis in hypothyroidism
Gut-centric interventions (probiotics, prebiotics, synbiotics (combination pro- and prebiotics), dietary fiber, nutrient-dense diet) hold promise as adjunctive support for hypothyroidism — potentially improving not just digestion but thyroid hormone regulation, inflammation, and overall metabolic health.
Because a dysbiotic gut microbiome may influence how well thyroid medications (like levothyroxine) are absorbed (especially in conditions like SIBO or slow gut motility), supporting gut health may also improve medication efficacy. Some functional-medicine practitioners argue that treating SIBO or gut dysbiosis can reduce required medication doses — though robust clinical trials are still lacking.
Given the evidence from animal models, influencing your gut microbiome might be more than “nice to have” — it might actually shift thyroid hormone dynamics. But more human trials are needed.
Strategies for dysbiosis during the menopausal transition
For perimenopausal or menopausal women, supporting gut health might ease some menopause-linked symptoms, like weight gain, metabolic slowdown, inflammation, and mood changes.
Effective approaches could include increasing dietary fiber and prebiotics, eating phytoestrogen-rich plant foods, and considering probiotic or synbiotic support (especially strains known to support estrogen metabolism or gut barrier integrity).
Because the gut microbiome shifts after menopause toward a “more male-like” composition, adopting lifestyle and diet strategies that support a diverse, estrogen-friendly microbiome might help counteract menopausal metabolic/immune changes.
GLP-1 drugs and the gut microbiome
Restoring gut microbial balance could improve metabolic flexibility, hormone regulation, inflammation, and energy handling — making weight-loss efforts more effective in the long-term.
A recent review (2024) found that GLP-1 drugs (widely used for weight loss and metabolic health) may help restore a healthier gut microbiome: increasing beneficial bacteria (e.g., Bacteroides, Lactobacillus, Bifidobacterium), reducing harmful species, improving gut barrier integrity, reducing systemic inflammation, and boosting SCFA production.
Because these drugs can influence gut–brain–metabolism signaling and microbial ecology, they may help “break the cycle” of dysbiosis, leading to poor metabolism, leading to weight gain, especially in people whose dysbiosis started as a result of hormonal dysfunction, gut permeability, or metabolic derangement.
If weight gain or difficulty losing weight is linked to gut dysbiosis (common in thyroid disorders or menopause), then interventions that target the gut (diet, fiber, pre/probiotics) may help — and in some cases, GLP-1 agonist therapy might work better if combined with efforts to support gut health.
Based on current evidence, here are practical, evidence-informed general strategies to support a resilient, balanced gut microbiome. These may benefit digestion, thyroid function, hormonal health, metabolic health, and overall wellbeing, as well as aid in weight loss.
Eat a varied, fiber-rich, plant-forward diet
- Aim for lots of fruits, vegetables, whole grains, legumes, nuts, and seeds — diversity matters. Different plant foods support different microbial species.
- Fiber and resistant starch (from foods like oats, beans, lentils, and greens) fuel beneficial gut bacteria and support the production of short-chain fatty acids (SCFAs), which help maintain gut integrity and regulate inflammation.
- Minimize ultra-processed foods, excessive saturated fat, or sugar, as these tend to promote dysbiosis and inflammation.

Include prebiotics and (if appropriate) probiotics/synbiotics
- Prebiotics (non-digestible fibers) support the growth of beneficial bacteria.
- Probiotics or synbiotics may help — especially if you have known gut issues (bloating, SIBO, irregularity), thyroid disease, or hormonal changes. Some early trials show promise for thyroid health.
- That said, probiotics are not one-size-fits-all. It’s best to choose strains backed by research and consider consulting a healthcare provider.
Support thyroid- and hormone-friendly nutrients
- Make sure you’re getting adequate iodine, selenium, zinc, and iron — all important for thyroid function and often affected by gut health.
- Also support general gut health with nutrients necessary for mucosal integrity (e.g., fiber, glutamine-rich foods, polyphenols, micronutrients).

Prioritize lifestyle factors: sleep, stress management, moderate exercise
- Stress, poor sleep, and a sedentary lifestyle can all negatively impact gut microbiome balance.
- Gentle exercise helps support healthy gut motility and microbial diversity; high-intensity or stressful exercise may be less beneficial if recovery is poor.
Support stable hormone & metabolic health
- For thyroid issues: work with your provider to optimize your thyroid hormone replacement treatment, but also look at gut health as an important complementary factor.
- For women in perimenopause or menopause: pay attention to diet, gut-supportive habits, and perhaps consider gut-focused interventions to ease hormonal and metabolic changes.
- For metabolic health and weight management: pair a healthy microbiome-supportive diet and lifestyle with mindful metabolism support; if medications like GLP-1 agonists are used, support the gut to improve outcomes.
Be patient and consistent — the gut microbiome doesn’t change overnight
- Restoring balance often takes time (weeks to months of consistent diet and lifestyle changes).
- Monitoring symptoms (digestion, energy, mood, metabolism) can help you track progress more meaningfully than obsessing over microbial “perfect balance.”
Expert recommendations
Dr. Kyle Hoedebecke, MD, MBA, MPA, MS, FAAFP, CPE, a Clinical Advisor at Alpas Wellness, has some thoughts about how to address imbalances in the gut and the microbiome.
“I recommend taking a holistic approach to health and focus on self-care that consists of a high-fiber plant-based diet, regular intake of fermented foods, healthy methods of stress reduction, and regular exercise. For women going through menopause, I advise you to pay special attention to supporting your estrobolome with phytoestrogens, which can help keep your microbiomes healthy. Both prebiotics and probiotics are important for resolving dysbiosis, since prebiotics act as a source of fiber that can feed existing beneficial bacteria within our microbiomes, while probiotics introduce new beneficial gut bacteria, to strongly support a balanced gut microbiome, in turn supporting overall health.”
Dr. Gerome Burke sums up the general recommendation, “starting with foundational lifestyle and dietary changes.” Dr. Burke recommends several ways to address gut dysbiosis in general:
- “Adopt a whole-foods diet that is rich in diverse fibers (e.g., green leafy vegetables, fruits, legumes, whole grains). These fibers act as “prebiotics,” which are beneficial for your gut bacteria.
- Reduce or eliminate inflammatory triggers that can push the body into an insulin-resistant state. (e.g., ultra-processed foods, refined sugars).
- Stress management is critically important, as chronic stress can directly alter your gut microbiome.
- Incorporating mindfulness, adequate sleep, and regular, moderate exercise supports the development of a healthier gut microbiome.
- Consulting with a healthcare provider for persistent adverse symptoms can help identify specific imbalances and rule out other conditions.”
Registered dietitian Alison Swiggard, D, recommends several self-care strategies to address gut dysbiosis in general.
“My first-line approach avoids restriction or extreme protocols. Instead, I focus on stabilizing the nervous system, increasing dietary variety, and supporting regularity. Some accessible approaches include:
- Eating every 3–4 hours to support the migrating motor complex and reduce stalling in the GI tract
- Gradually increasing fiber diversity, especially through plants with different textures and colors
- Ensuring adequate hydration and gentle movement to support motility
- Incorporating stress-reducing practices (breathwork, grounding, rest), since chronic stress alone can shift the microbiome
- Limiting excessive alcohol and avoiding unnecessary elimination diets unless guided by a clinician
These strategies tend to be more sustainable and protective of long-term gut health than harsh cleanses or restrictive protocols.”
.webp)
Future research needs in this area are becoming increasingly clear. One major challenge is that a “healthy microbiome” is not one-size-fits-all. There is no universal definition of a perfect gut ecosystem; what constitutes a healthy microbiome likely varies based on an individual’s genetics, hormones, diet, age, and overall physiology. Much of the existing research focuses on correlations rather than clear cause-and-effect relationships, making it difficult to translate findings into personalized clinical guidance.
Another important gap is the limited number of robust intervention studies. While some clinical trials suggest that probiotics, synbiotics, or targeted dietary changes may offer benefits, we still lack large, long-term randomized controlled trials. This gap is particularly notable for populations such as people with autoimmune thyroid disease, those going through menopause, or individuals with metabolic dysfunction, who may have unique microbiome–hormone interactions that are not well represented in current research.
Microbiome–medication interactions also remain underexplored. A 2025 press release from the Endocrine Society suggested that appropriate thyroid hormone treatment may reduce the risk of small intestinal bacterial overgrowth (SIBO) in people with hypothyroidism, hinting at a meaningful connection between endocrine therapy and gut health. However, much more research is needed to understand how treatments like thyroid hormone replacement, GLP-1 medications, hormone replacement therapy, and other interventions shape the microbiome—and how the microbiome, in turn, influences the effectiveness and side effects of these therapies.
Finally, the crosstalk between hormones, the microbiome, and the immune system is deeply complex and only beginning to be understood. The ways in which gut microbes interact with sex hormones, immune signaling, and thyroid regulation likely play a significant role in health and disease, but the mechanisms behind these interactions remain largely uncharted territory for future research.
At Paloma, we recognize that gut dysbiosis is often not just a side issue—it can be a key driver behind persistent thyroid symptoms, hormonal imbalance, and frustrating weight loss resistance. Growing research shows that disruptions in the gut microbiome may contribute to the development or worsening of Hashimoto’s disease and hypothyroidism, as well as to the metabolic and inflammatory changes seen during perimenopause and menopause. That’s why addressing gut health alongside hormone optimization can be such a powerful, complementary strategy.
Paloma’s approach for members is designed to support the whole picture. Our thyroid-focused care helps optimize hormone replacement and monitoring with convenient home test kits, while also acknowledging how digestion, nutrient absorption, inflammation, and gut integrity affect how you feel—and how well treatments work. For women navigating perimenopause or menopause, we help address the interconnected shifts in thyroid function, weight, energy, and gut health that so often occur together.
We also support patients working toward sustainable weight loss, including those using GLP-1 medications. Emerging evidence suggests that GLP-1 therapies may positively influence the gut microbiome, and outcomes may be even better when paired with gut-supportive nutrition, fiber intake, and lifestyle strategies. Whether weight challenges are driven by thyroid dysfunction, hormonal changes, dysbiosis, or a combination of all three, Paloma helps you address the underlying biology—not just the number on the scale.
By integrating thyroid care with a deeper understanding of gut health, metabolism, and hormonal transitions, Paloma empowers you with evidence-based guidance, personalized support, and a compassionate path forward—so your body can heal, respond, and thrive more effectively.
- Gut dysbiosis, an imbalance in the gut microbiome, disrupts digestion, immune system function, metabolism, and hormone regulation.
- The gut-thyroid axis links gut health to thyroid disorders such as Hashimoto’s thyroiditis and hypothyroidism, with altered microbiota composition and increased intestinal permeability commonly found.
- Hormonal fluctuations during perimenopause and menopause significantly impact the gut microbiome, affecting estrogen metabolism, chronic inflammation, and metabolic health.
- Leaky gut and chronic inflammation stemming from dysbiosis can fuel autoimmune activity and contribute to systemic hormonal disruption.
- An imbalanced gut microbiome makes weight loss more challenging by exacerbating insulin resistance and disrupting appetite-regulating hormones.
- Supporting gut health through an anti-inflammatory diet, adequate fiber intake, targeted prebiotics/probiotics, and lifestyle modifications is crucial for thyroid health, hormonal balance, and metabolic wellbeing.

.webp)











